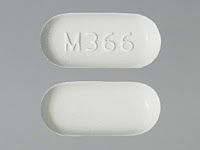
What is Hydrocodone ?
What is Hydrocodone ?
Preceding recommending hydrocodone, survey every patient's hazard for narcotic habit, misuse, and abuse, and normally screen all patients for narcotic fixation practices or conditions. To guarantee that the advantages of narcotic analgesics exceed the dangers of compulsion, misuse, and abuse, the Food and Drug Administration (FDA) has required a Risk Evaluation and Mitigation Strategy (REMS) for these items. Screen for respiratory sorrow, which can be not kidding, hazardous, or lethal, particularly during commencement or following a portion increment. Educate patients to gulp down stretched out discharge structures to keep away from conceivably deadly overdose.
Unintentional ingestion of hydrocodone can bring about a lethal overdose, particularly in kids. Whenever delayed narcotic use is essential during pregnancy, instruct patient concerning potential danger of hazardous neonatal narcotic withdrawal disorder and guarantee suitable treatment will be accessible. Use with CYP3A4 inhibitors or inducers may change hydrocodone plasma levels bringing about delayed unfavorable impacts or possibly deadly respiratory sorrow; observing is suggested. Associative utilization of narcotics with benzodiazepines or different CNS depressants, including liquor, may bring about significant sedation, respiratory melancholy, extreme lethargies, and demise. Save attendant endorsing for patients with insufficient elective treatment alternatives,
 limit measurements and term to the base required, and screen for respiratory melancholy and sedation. Mixed drinks or prescriptions containing liquor ought to be stayed away from because of the potential for expanded hydrocodone plasma levels and a conceivably deadly overdose.Addiction, Abuse, and MisuseHydrocodone broadened discharge (ER) uncovered patients and different clients to the dangers of narcotic dependence, misuse, and abuse, which can prompt overdose and passing. Survey every patient's hazard before endorsing Hydrocodone ER, and screen all patients routinely for the improvement of these practices or conditions.Opioid Analgesic Risk Evaluation and Mitigation Strategy (REMS)To guarantee that the advantages of narcotic analgesics exceed the dangers of habit, misuse and abuse, the Food and Drug Administration (FDA) has required a REMS for these items. Under the necessities of the REMS, sedate organizations with affirmed narcotic pain relieving items must make REMS-agreeable instruction programs accessible to human services suppliers.
limit measurements and term to the base required, and screen for respiratory melancholy and sedation. Mixed drinks or prescriptions containing liquor ought to be stayed away from because of the potential for expanded hydrocodone plasma levels and a conceivably deadly overdose.Addiction, Abuse, and MisuseHydrocodone broadened discharge (ER) uncovered patients and different clients to the dangers of narcotic dependence, misuse, and abuse, which can prompt overdose and passing. Survey every patient's hazard before endorsing Hydrocodone ER, and screen all patients routinely for the improvement of these practices or conditions.Opioid Analgesic Risk Evaluation and Mitigation Strategy (REMS)To guarantee that the advantages of narcotic analgesics exceed the dangers of habit, misuse and abuse, the Food and Drug Administration (FDA) has required a REMS for these items. Under the necessities of the REMS, sedate organizations with affirmed narcotic pain relieving items must make REMS-agreeable instruction programs accessible to human services suppliers.
Medicinal services suppliers are emphatically urged to: complete a REMS-agreeable instruction program, counsel patients and additionally their guardians, with each solution, on safe use, genuine dangers, stockpiling, and transfer of these items, underscore to patients and their parental figures the significance of perusing the Medication Guide each time it is given by their drug specialists, and think about different apparatuses to improve patient, family unit, and network safety.Life-Threatening Respiratory DepressionSerious, hazardous, or lethal respiratory despondency may happen with utilization of Hydrocodone ER. Screen for respiratory sorrow, particularly during commencement of Hydrocodone ER or following a portion increment. Train patients to gulp down hydrocodone bitartrate ER tablets; squashing, biting, or dissolving hydrocodone bitartrate tablets can cause fast discharge and retention of a possibly lethal portion of hydrocodone bitartrate.Accidental IngestionAccidental ingestion of even one portion of Hydrocodone ER, particularly by youngsters, can bring about a deadly overdose of Hydrocodone ER.Neonatal Opioid Withdrawal
 SyndromeProlonged utilization of Hydrocodone ER during pregnancy can bring about neonatal narcotic withdrawal disorder, which might be hazardous if not perceived and treated, and requires the executives as indicated by conventions created by neonatology specialists. On the off chance that narcotic use is required for a drawn out period in a pregnant lady, educate the patient regarding the danger of neonatal narcotic withdrawal disorder and guarantee that proper treatment will be available.Cytochrome P450 3A4 InteractionThe corresponding utilization of Hydrocodone ER with all cytochrome P450 3A4 inhibitors may bring about an expansion in Hydrocodone plasma fixations, which could increment or delay antagonistic responses and may cause possibly lethal respiratory gloom. Likewise, end of a correspondingly utilized cytochrome P450 3A4 inducer may bring about an expansion in Hydrocodone plasma focus. Screen patients getting Hydrocodone ER and any CYP3A4 inhibitor or inducer.Risks from Concomitant Use with Benzodiazepines or Other CNS DepressantsReserve attendant endorsing of Hydrocodone ER and benzodiazepines or different CNS depressants for use in patients for whom elective treatment choices are lacking. Point of confinement measurements and lengths to the base required. Pursue patients for signs and side effects of respiratory melancholy and sedation
SyndromeProlonged utilization of Hydrocodone ER during pregnancy can bring about neonatal narcotic withdrawal disorder, which might be hazardous if not perceived and treated, and requires the executives as indicated by conventions created by neonatology specialists. On the off chance that narcotic use is required for a drawn out period in a pregnant lady, educate the patient regarding the danger of neonatal narcotic withdrawal disorder and guarantee that proper treatment will be available.Cytochrome P450 3A4 InteractionThe corresponding utilization of Hydrocodone ER with all cytochrome P450 3A4 inhibitors may bring about an expansion in Hydrocodone plasma fixations, which could increment or delay antagonistic responses and may cause possibly lethal respiratory gloom. Likewise, end of a correspondingly utilized cytochrome P450 3A4 inducer may bring about an expansion in Hydrocodone plasma focus. Screen patients getting Hydrocodone ER and any CYP3A4 inhibitor or inducer.Risks from Concomitant Use with Benzodiazepines or Other CNS DepressantsReserve attendant endorsing of Hydrocodone ER and benzodiazepines or different CNS depressants for use in patients for whom elective treatment choices are lacking. Point of confinement measurements and lengths to the base required. Pursue patients for signs and side effects of respiratory melancholy and sedation
.
Relatrd Articles :
Preceding recommending hydrocodone, survey every patient's hazard for narcotic habit, misuse, and abuse, and normally screen all patients for narcotic fixation practices or conditions. To guarantee that the advantages of narcotic analgesics exceed the dangers of compulsion, misuse, and abuse, the Food and Drug Administration (FDA) has required a Risk Evaluation and Mitigation Strategy (REMS) for these items. Screen for respiratory sorrow, which can be not kidding, hazardous, or lethal, particularly during commencement or following a portion increment. Educate patients to gulp down stretched out discharge structures to keep away from conceivably deadly overdose.
Unintentional ingestion of hydrocodone can bring about a lethal overdose, particularly in kids. Whenever delayed narcotic use is essential during pregnancy, instruct patient concerning potential danger of hazardous neonatal narcotic withdrawal disorder and guarantee suitable treatment will be accessible. Use with CYP3A4 inhibitors or inducers may change hydrocodone plasma levels bringing about delayed unfavorable impacts or possibly deadly respiratory sorrow; observing is suggested. Associative utilization of narcotics with benzodiazepines or different CNS depressants, including liquor, may bring about significant sedation, respiratory melancholy, extreme lethargies, and demise. Save attendant endorsing for patients with insufficient elective treatment alternatives,
 limit measurements and term to the base required, and screen for respiratory melancholy and sedation. Mixed drinks or prescriptions containing liquor ought to be stayed away from because of the potential for expanded hydrocodone plasma levels and a conceivably deadly overdose.Addiction, Abuse, and MisuseHydrocodone broadened discharge (ER) uncovered patients and different clients to the dangers of narcotic dependence, misuse, and abuse, which can prompt overdose and passing. Survey every patient's hazard before endorsing Hydrocodone ER, and screen all patients routinely for the improvement of these practices or conditions.Opioid Analgesic Risk Evaluation and Mitigation Strategy (REMS)To guarantee that the advantages of narcotic analgesics exceed the dangers of habit, misuse and abuse, the Food and Drug Administration (FDA) has required a REMS for these items. Under the necessities of the REMS, sedate organizations with affirmed narcotic pain relieving items must make REMS-agreeable instruction programs accessible to human services suppliers.
limit measurements and term to the base required, and screen for respiratory melancholy and sedation. Mixed drinks or prescriptions containing liquor ought to be stayed away from because of the potential for expanded hydrocodone plasma levels and a conceivably deadly overdose.Addiction, Abuse, and MisuseHydrocodone broadened discharge (ER) uncovered patients and different clients to the dangers of narcotic dependence, misuse, and abuse, which can prompt overdose and passing. Survey every patient's hazard before endorsing Hydrocodone ER, and screen all patients routinely for the improvement of these practices or conditions.Opioid Analgesic Risk Evaluation and Mitigation Strategy (REMS)To guarantee that the advantages of narcotic analgesics exceed the dangers of habit, misuse and abuse, the Food and Drug Administration (FDA) has required a REMS for these items. Under the necessities of the REMS, sedate organizations with affirmed narcotic pain relieving items must make REMS-agreeable instruction programs accessible to human services suppliers.Medicinal services suppliers are emphatically urged to: complete a REMS-agreeable instruction program, counsel patients and additionally their guardians, with each solution, on safe use, genuine dangers, stockpiling, and transfer of these items, underscore to patients and their parental figures the significance of perusing the Medication Guide each time it is given by their drug specialists, and think about different apparatuses to improve patient, family unit, and network safety.Life-Threatening Respiratory DepressionSerious, hazardous, or lethal respiratory despondency may happen with utilization of Hydrocodone ER. Screen for respiratory sorrow, particularly during commencement of Hydrocodone ER or following a portion increment. Train patients to gulp down hydrocodone bitartrate ER tablets; squashing, biting, or dissolving hydrocodone bitartrate tablets can cause fast discharge and retention of a possibly lethal portion of hydrocodone bitartrate.Accidental IngestionAccidental ingestion of even one portion of Hydrocodone ER, particularly by youngsters, can bring about a deadly overdose of Hydrocodone ER.Neonatal Opioid Withdrawal
 SyndromeProlonged utilization of Hydrocodone ER during pregnancy can bring about neonatal narcotic withdrawal disorder, which might be hazardous if not perceived and treated, and requires the executives as indicated by conventions created by neonatology specialists. On the off chance that narcotic use is required for a drawn out period in a pregnant lady, educate the patient regarding the danger of neonatal narcotic withdrawal disorder and guarantee that proper treatment will be available.Cytochrome P450 3A4 InteractionThe corresponding utilization of Hydrocodone ER with all cytochrome P450 3A4 inhibitors may bring about an expansion in Hydrocodone plasma fixations, which could increment or delay antagonistic responses and may cause possibly lethal respiratory gloom. Likewise, end of a correspondingly utilized cytochrome P450 3A4 inducer may bring about an expansion in Hydrocodone plasma focus. Screen patients getting Hydrocodone ER and any CYP3A4 inhibitor or inducer.Risks from Concomitant Use with Benzodiazepines or Other CNS DepressantsReserve attendant endorsing of Hydrocodone ER and benzodiazepines or different CNS depressants for use in patients for whom elective treatment choices are lacking. Point of confinement measurements and lengths to the base required. Pursue patients for signs and side effects of respiratory melancholy and sedation
SyndromeProlonged utilization of Hydrocodone ER during pregnancy can bring about neonatal narcotic withdrawal disorder, which might be hazardous if not perceived and treated, and requires the executives as indicated by conventions created by neonatology specialists. On the off chance that narcotic use is required for a drawn out period in a pregnant lady, educate the patient regarding the danger of neonatal narcotic withdrawal disorder and guarantee that proper treatment will be available.Cytochrome P450 3A4 InteractionThe corresponding utilization of Hydrocodone ER with all cytochrome P450 3A4 inhibitors may bring about an expansion in Hydrocodone plasma fixations, which could increment or delay antagonistic responses and may cause possibly lethal respiratory gloom. Likewise, end of a correspondingly utilized cytochrome P450 3A4 inducer may bring about an expansion in Hydrocodone plasma focus. Screen patients getting Hydrocodone ER and any CYP3A4 inhibitor or inducer.Risks from Concomitant Use with Benzodiazepines or Other CNS DepressantsReserve attendant endorsing of Hydrocodone ER and benzodiazepines or different CNS depressants for use in patients for whom elective treatment choices are lacking. Point of confinement measurements and lengths to the base required. Pursue patients for signs and side effects of respiratory melancholy and sedation.
Relatrd Articles :
Comments
Post a Comment